Pool Chemistry Guide & Water Chemicals Chart
Dec 30, 2017
There are a variety of situations that often distract pool owners, like algae bloom, swimmer's ear, stinging eyes, and irritated skin. The use of the proper pool chemicals in the right concentrations certainly helps. However, how much is enough? How much is too much?
On average, pool owners spend $87 per month on chemicals. In most cases, that is way more than necessary. Too often, chemical applications are imprecise at best and haphazard at worst - avoidable waste is all too common. For most pool owners, time is also a valuable commodity, especially during the coveted swimming season.
Unnecessary pool maintenance wastes time and money, which is not what you had in mind when you enthusiastically purchased or built a home with a pool. Those inviting waters are for pleasure and perhaps some exercise. Maintaining your pool should not be like a second job. Also, it should not be an unnecessary drain on the wallet.
The guide that follows includes dosage charts, which specify exact amounts of pool chemicals for different situations. Consult our comprehensive guide time and again to get the pool chemistry information you need. Spend less time maintaining your pool and more time enjoying it. Whatever pool chemistry challenges you face, you will find the timesaving and cost-saving answers right here. There is no need to “re-invent” the subject of pool chemistry - we've already done the heavy lifting for you!
It is time to reinvigorate the dream - to restore and maintain your pool as the centerpiece of family camaraderie and neighborhood fun you once envisioned. Get back to hosting memorable parties, birthday bashes and far more - all with minimal hassle.
CONTENTS

OVERVIEW
Below are key steps to keeping your pool in tip-top shape - including getting rid of debris, setting up and using the vacuum, backwashing and cleaning the pump filter and adjusting the chemistry. Many pool owners find that it is possible to complete routine pool maintenance in an hour or less, leaving more time to kick back, relax and enjoy it all.
It is important to keep debris from building up, and the pool should be vacuumed periodically. Before vacuuming, it is helpful to direct the return jets downward to minimize surface ripples. This makes it easier to see the bottom of the pool. Carefully overlapping passes is a key to success. It takes about a half-hour to vacuum a typical outdoor pool. Finally, brush any algae from the sides of the pool.
The filter should be backwashed frequently enough to allow the pump to freely circulate and filter the water. The pressure gauge present on most systems lets you know when backwashing is necessary. Pressures 8-10 lbs. higher than start-up levels signal a need for backwashing. With a DE system, backwashing sends material to a filter bag. Cartridges in those kinds of systems can simply be hosed off and re-used. Also, hair and lint caught in the pump filter needs to be removed on a regular basis.
Finally, it is important to periodically test for chlorine, pH, calcium hardness and cyanuric acid (CYA).
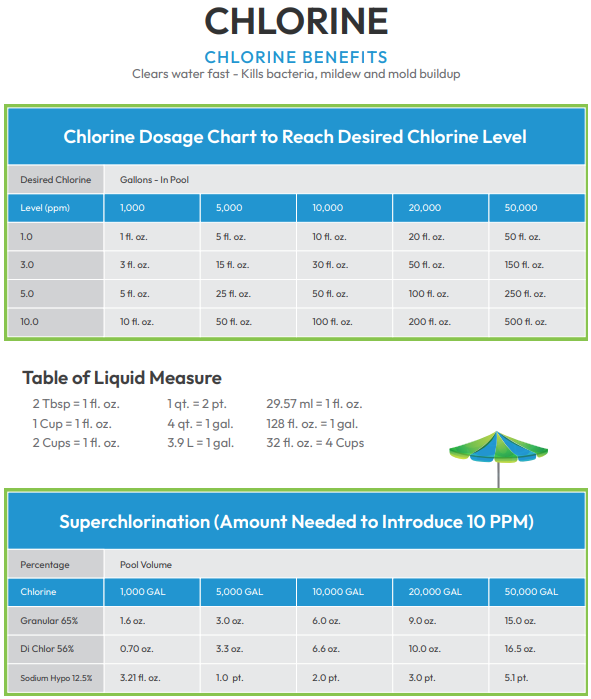
CHLORINE
First, it is important to distinguish between free chlorine (FC) and combined chlorine (CC). It is important to have enough free chlorine in the pool to treat organic impurities. When FC binds to these elements, it becomes combined chlorine. For example, in the right concentrations, FC prevents algae blooms. FC must be constantly replenished, or you risk an infestation of algae. It is vital to have adequate levels of FC to maintain a pool full of clear, sanitary and chemically balanced water.
Free Chlorine - Use a salt-water chlorine generator (SWCG) to reduce FC testing intervals from once a day to once every few days. The typical tube-like system allows chlorine salts from sticks to continually replenish FC levels. The addition of chlorine-laden salts also softens the water. Appropriate salt levels in the pool are modest - typically less than 10 percent of that found in seawater. Most swimmers cannot taste salt such levels. The SWCG cell should be examined once a year to confirm that it is free of debris and that the plates are still metallic or black in appearance. Each cell will typically last 3-5 years.
Combined Chlorine - The chemical reaction between free chlorine and organic matter like algae, sweat and urine produces combined chlorine. CC produces chloramines, which emit the characteristic “chlorine” odor. Other than the distracting odor, chloramines are also associated with respiratory problems like asthma. The interaction of free chlorine and organic substances may also produce potentially carcinogenic nitrosamines. Therefore, it is important to employ proactive measures to reduce algae bloom and bacteria growth in the first place.
Algae bloom can be a real problem. One of the more high-profile cases of algae bloom occurred at the 2016 Rio Olympics. The water in the diving pool turned green before the women’s synchronized diving final, according to Time. The unwanted contrast with the clear blue waters of the adjacent lap pool was dramatic, and the murky green waters had to be cleansed of algae before competition continued. Officials later explained that certain tanks holding pool chemicals ran dry.
The amount of sunlight exposure and cyanuric acid (CYA) help to determine how much free chlorine your pool requires. Cyanuric Acid, a water stabilizer/conditioner, reduces the damaging effect of the sun’s UV rays on free chlorine. Ultimately, maintaining the right levels of both FC and CYA will yield sanitized, healthy water without unnecessarily wasting time or money. Although it is also possible to sanitize an outdoor pool with bromine, it is less stable in sunlight, and it is more expensive.
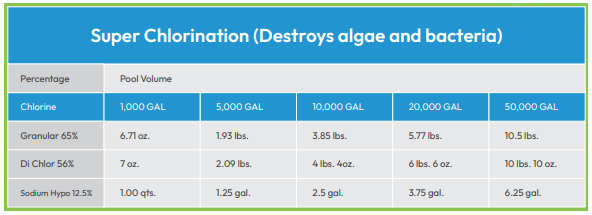
When CC levels exceed 0.5 ppm, it is time to “shock” your pool. For example, if an algae bloom occurs, eradicate it by shocking, or hyper-chlorinating your pool. Since this process of killing excess, unwanted organic matter can take days, it’s important to do it enough but not too much.
PH - ACIDITY & ALKALINITY
Pool water that is either too acidic or too alkaline (base) causes problems. The full pH scale extends from 0 to 14.0. Acidic pH levels are at the lower end of the scale, and alkaline levels are at the upper end. The 7.0 midpoint represents a balance between the two. Not surprisingly, pure distilled water has a pH right at this midpoint.
For swimmers, the ideal pH range is slightly alkaline - usually between 7.2 and 7.8, according to the Centers for Disease Control and Prevention. Why does this matter? First of all, a pH level below 7.2 may sting the eyes or irritate the skin. Acidic pH levels below 6.8 can even etch or corrode metal components in pool equipment. Acidic water may also damage the lining of a pool over time. Acidic pool water concerned a group of dental school researchers enough that they examined its impact on dental enamel. High pH levels are also a problem because they may cause calcium scaling or cloudy water. Also, chlorine is less effective in water with a pH higher than 7.8.
Fortunately, it is often possible to maintain swimmer health and comfort and reduce equipment wear by simply maintaining proper pH levels in your pool. However, these levels naturally fluctuate or “drift” for a number of reasons:
- Water temperature fluctuations
- Aeration from rain or waterfall
- Leaching of fresh plaster in a new pool
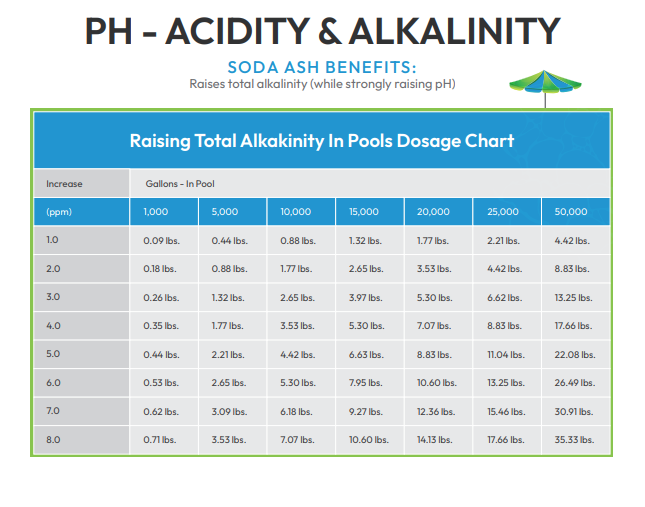
Once a pattern of pH drift is fully understood, less testing is needed. When necessary, it is possible to lower pH levels with a reducer like muriatic acid and to raise them with an increaser like soda ash or borax. Acid or base demand testing allows you to determine the amounts required.
Total Alkalinity
There are important reasons for maintaining proper TA levels, so regular testing is recommended. According to the American Chemistry Council website, the Association of Pool and Spa Professionals (APSP) recommends that total alkalinity (TA) in swimming pools remain between 80 and 120 ppm.
Low TA - When TA is too low, it is time to add sodium bicarbonate (baking soda), soda ash or sodium sesquicarbonate. The use of traditional baking soda is the most common way to raise total alkalinity. If remedial action is not taken, low TA may cause:
- pH bounce - rapidly changing pH levels
- Stinging eyes
- Staining of pool walls and floor
- Corroding metal
- Green water
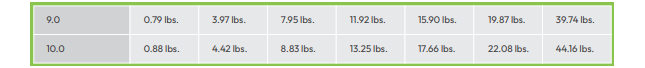
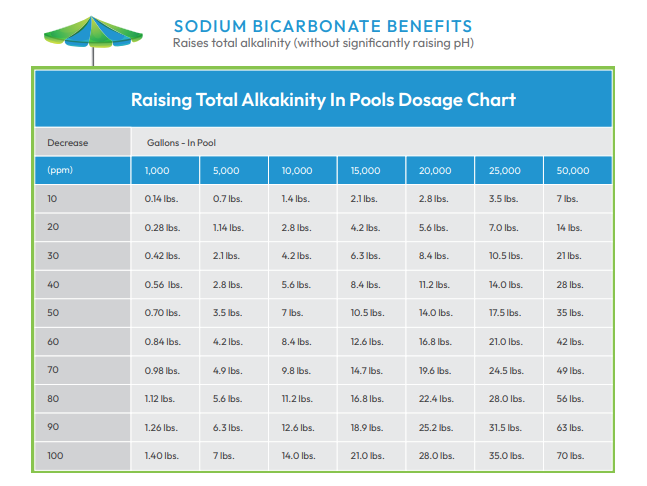
It may take several days to raise TA levels, because there are limits to how fast sodium bicarbonate can be safely added to the pool water. Therefore, it is ideal to keep levels within the acceptable range as much as possible.
High TA - TA levels that are too high are also a problem. High TA results in:
- Reduced chlorine effectiveness
- Cloudy water
- Repeated efforts to lower pH
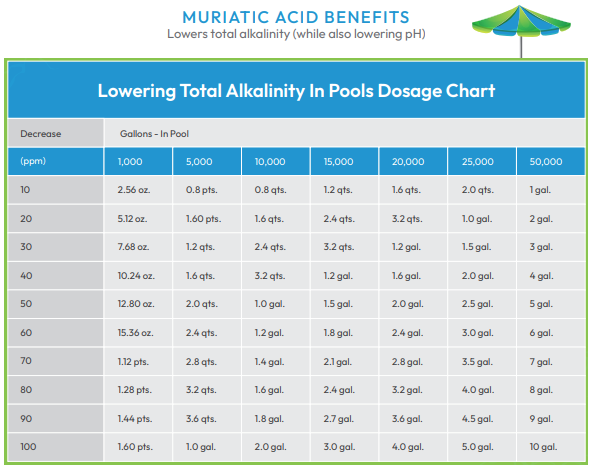
Increasing total alkalinity is a time-consuming process, so it is ideal to maintain TA within the acceptable range as much as possible. Fortunately, in a well-maintained pool, TA levels tend to remain fairly stable.
PHOSPHATES & ALGAE
Because phosphates (PO4) are such good plant nutrients, farmers add them to their fields to increase crop yields. Unfortunately, when sufficient quantities of PO4 find their way into pools, algae blooms are common.
As a result, periodic testing for phosphates is important. When phosphate levels are unacceptable, it is important to identify the source(s) in order to reduce or eliminate them. Phosphate removers are a potential solution, although they must be used properly. Research has verified the link between the presence of phosphates and algae growth. In a study at Grinnell College in Iowa, researchers confirmed that phosphates stimulate unwanted algae bloom.
How do phosphates get into pool water in the first place? There are a variety of suggested sources, including source water, fertilizers in runoff, municipal water treated with polyphosphates and windblown dirt, dust and organic debris.
Fortunately, many of these sources do not always have a significant impact on phosphate levels. For example, phosphate levels of source water are usually too low (about 20-100 ppb) to stimulate algae bloom. In the past, polyphosphates were added to municipal water to reduce pipe corrosion, although this practice is no longer common. Only limited phosphates ride along on airborne dirt and debris. However, phosphates in stain/scale products may become a problem, so use low-phosphate compounds to cope with pool stains and scaling.
Note: You can maintain pH and keep chlorine in the pool and still have high levels of phosphate. Phosphates come in the source water, airborne, in chemicals and from swimmer waste. While maintaining pH and chlorine levels may help keep algae at bay. One hot day and a drop in chlorine and algae spores can bloom rapidly and be very resistant. Also, high levels of phosphate with high levels of calcium will cause the formation of calcium phosphate scale which proper pH and chlorine can do nothing to prevent.
CALCIUM HARDNESS
According to the APSP, calcium levels of 200-400 ppm are ideal. It is usually unnecessary to test for calcium hardness as often as pH and alkalinity. However, this does not mean that calcium levels are not that important. Since both pH and TA impact calcium hardness, all three levels must be in balance to optimize pool conditions and reduce pool chemistry expenses long-term.
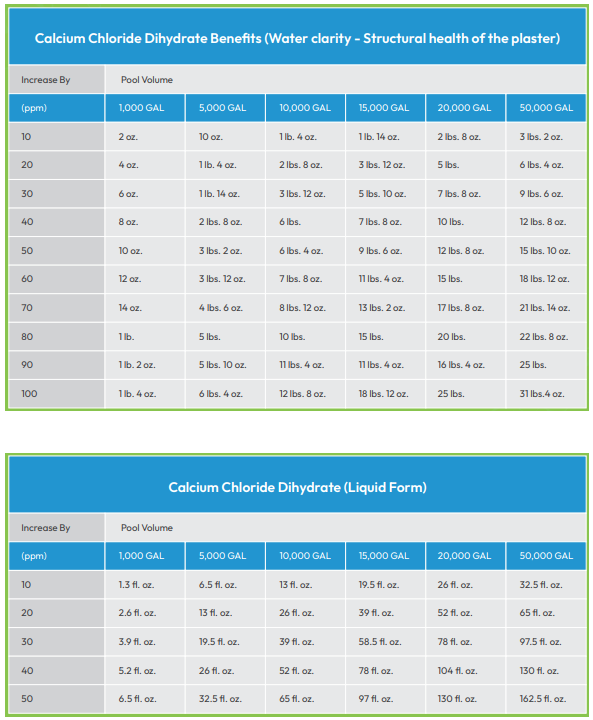
Too hard - Excessive calcium causes unwanted scaling or buildup on the sides of the pool, in pipes and in pool equipment. When calcium exceeds 400 ppm, levels must be reduced, often through the use of a flocculent. This compound causes calcium clumping, making it easier to remove. After application, it is necessary to backwash the pump, clean the pump filter and brush calcium buildup off the sides and floor of the pool. If your tap water is not too hard, calcium reduction is possible by simply draining away some of the pool water and replacing it.
Too soft - Pool water that is too soft is also a problem. When calcium levels slip below 150 ppm, acidic pool water can etch or even dissolve concrete. It can also corrode the metal components of pool equipment. To correct this, simply add calcium chloride to restore calcium to appropriate levels.
CYANURIC ACID
Cyanuric acid (CYA) is popular in outdoor pools because it bonds with chlorine molecules to make them less vulnerable to the sun's ultraviolet (UV) rays. Without CYA, bright sunshine can cut chlorination by 90 percent in just two hours, according to sciencing.com. The APSP recommends CYA levels of 30-50 ppm in outdoor pools to keep chlorine depletion at a minimum.
Low CYA - Raise low CYA levels by adding the right amount of pool conditioner or stabilizer. Pool owners typically add CYA delivered in granular form. Correct calculations are a must because high CYA concentrations are more difficult to correct.
High CYA - Excessive CYA concentrations cause a variety of problems. For example, too much CYA actually reduces chlorine’s effectiveness in battling bacteria and organic matter. Once cyanuric acid levels get too high, the only real solution is dilution. After the percentage of excess CYA is calculated, that amount of pool water must be drained away and replaced with fresh water. For example, a CYA level that is 10 percent too high requires the replacement of one tenth of the water in the pool.
Some CYA corrections occur naturally. For example, rainfall will gradually reduce CYA levels through dilution. Splash out and backwashing also dilute pool water, thus bringing high levels of CYA under control.
Ultimately, CYA teams up with chlorine to cleanse, disinfect and stabilize pool water.
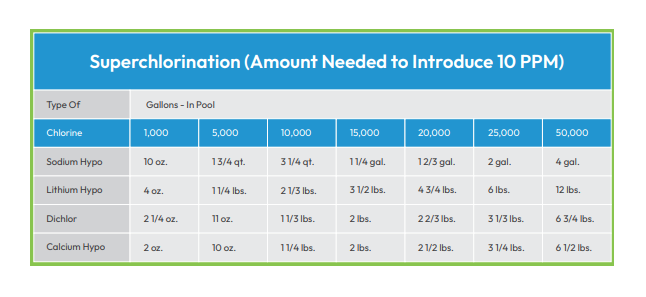
“Shocking” a pool is the process of adding large amounts of disinfectant in order to correct significant problems with contamination. There are both chlorine and non-chlorine shocks.
Chlorine - Shocking your pool with chlorine raises the disinfectant to very high levels. This destroys contaminants which have accumulated in the water, including ammonia, organic matter and nitrogen-containing compounds. Once this occurs, it is not safe to swim again until chlorine levels have dropped back down to 3-4 ppm. Testing confirms when it is safe to return to the water. Also, solar blankets and pool covers should not be used until chlorine levels drop to 3-4 ppm.
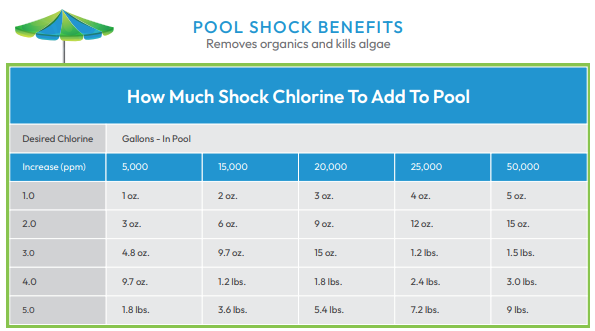
Non-chlorine - When you do not want to wait too long to return to swimming, shocking with potassium monopersulfate is an alternative. Keep in mind that this chemical neutralizes contaminants but it does not kill bacteria like chlorine does.
Although excess shocking wastes money and reduces pool availability, there are times when it is a good idea. First of all, is often appropriate to shock your pool at both the beginning and the end of the season. Early season shocking nips a springtime algae bloom in the bud.
Extreme weather conditions often warrant shocking. For example, during a heat wave, chlorine's disinfecting ability is reduced at the very same time that bacteria thrive. Also, very heavy rains may simultaneously unleash contaminants and increase pH.
Shocking your pool is also important when combined chlorine (CC) exceeds 0.5 ppm or when free chlorine (FC) drops to zero. When available FC is exhausted, that distinctive chlorine odor appears. The smell is caused by FC bonding with organic matter to create chloramines. This may occur after a big pool party, when sweat, pee and even skin moisturizers combine to dramatically raise the level of organic substances present in the water.
Precisely follow instructions on the bag of shock. Brush the pool with the pump running to better spread the chlorine. In windy conditions, only empty the bag up-wind, never into the wind. The best results occur when pH is between 7.2 and 7.4, or at least between 7.2 and 7.8.
CONCLUSION
When pool chemistry gets seriously out of whack, getting back on track may seem a little daunting at first. Water that turns green or cloudy is unsightly and often unsafe. However, fear not! Consult our charts to quickly identify the best solutions for your specific situation. We've demystified pool chemistry for once and for all.
You'll find that pool maintenance has never been easier - or less expensive!


